Exam 4 - Thermodynamics
What the Student Brings to Exams
- official UT ID card (with your picture and name on it)
- a simple scientific calculator (not a graphing calculator)
- a pencil(s) and eraser
- memorized formulas in your head - not on paper or anything else
- nothing else is allowed
What we provide for the Exams
- A printed copy of the exam (every exam has a unique version number on it).
- An answer sheet for the exam. This is a bubblesheet for your answers.
- An exam cover page that has ALL needed conversion factors and data. No formulas will be given.
- A periodic table of the elements with symbols, atomic number, and atomic weights.
What Formulas the Student Should Memorize
ΔU = Uf - Ui
ΔU = q + w
q = m Cs ΔT
q = n Cm ΔT
q = m ΔHtrans
q = n ΔHtrans
w = -PextΔV
w = -ΔngasRT
Δngas = (#mol gas prod) - (#mol gas react)
H = U + PV
ΔH = ΔU + PΔV
ΔU = ΔH - PΔV
ΔU = ΔH - ΔnRT
ΔH = qP
ΔU = qV
q = nRT ln(V2/V1)
w = –nRT ln(V2/V1)
qcal = -qsys
qcal = CcalΔT
qcal = mwater · Cs,water · ΔT + Chardware · ΔT
ΔHrxn = ΔH1 + ΔH2 + ΔH3 + ...
ΔHrxn° = ΣnΔHf° (products) - ΣnΔHf° (reactants)
ΔHrxn° ≈ ΣnΔHbond°(breaking) - ΣnΔHbond°(making)
ΔSuniv = ΔSsys + ΔSsurr
S = k ln Ω
ΔS = qrev / T
ΔS = n Cp ln(Tf / Ti)
ΔS = nR ln(V2/V1)
ΔStrans = ΔHtrans / Ttrans
ΔSrxn° = ΣnS° (products) - ΣnS° (reactants)
G = H - TS
ΔG = ΔH - TΔS
ΔGrxn° = ΣnΔGf° (products) - ΣnΔGf° (reactants)
ΔH = TeqΔS
What we provide on the exam cover page
We will provide all data for the exam. Specifically, we will provide a table of thermodynamic values, a table of phase change data, and a table of bond energies. We will also provide values for any constants and conversions needed.
Here is what the bond energy tables look like...
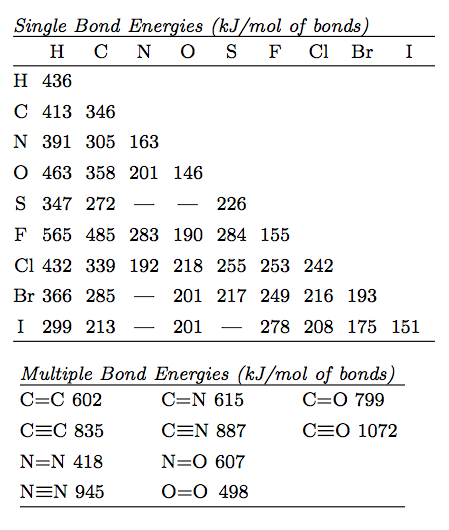
Here is what the thermo tables will look like - although different substances will be provided...
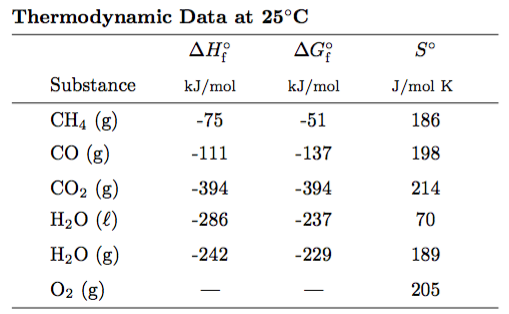
We might even add molar heat capacities (CP,m) to this table as well. And by-the-way... CP,m is the molar heat capacity at constant pressure.
The Bottom Line...
We will provide the data in some form of table. Be prepared to use any such table of data.